Dr Cindy Chu

Contact information
Borders


Cindy Chu
Senior clinical researcher
- LOMWRU paediatric pneumonia team lead
Bio
Cindy Chu is an internal medicine and paediatrics (IM/Paeds) physician working in the southeast Asia region since 2006. Her humanitarian work supporting clinical residency training in Lao PDR led her to the Mahidol Oxford Tropical Medicine Research Unit (MORU) at Shoklo Malaria Research Unit (SMRU) in Thailand where she began conducting Plasmodium vivax treatment trials under the tutelage of Professors Sir Nicholas White and François Nosten. She is now based at the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit (LOMWRU) in Lao PDR.
Her initial research focused on optimising the efficacy and effectiveness of primaquine (an 8-aminoquinoline prescribed for 7 to 14 days) for Plasmodium vivax radical cure and the intersection with glucose-6-phosphate dehydrogenase (G6PD) status.
Since the regulatory approval of tafenoquine, a single-dose 8-aminoquinoline agent, her research has shifted towards optimising tafenoquine dosing and expanding tafenoquine's role in P. vivax radical cure for patients of all ages. This aligns with her aspiration to improve health in populations in low middle income countries by improving health care delivery.
SEADOT study
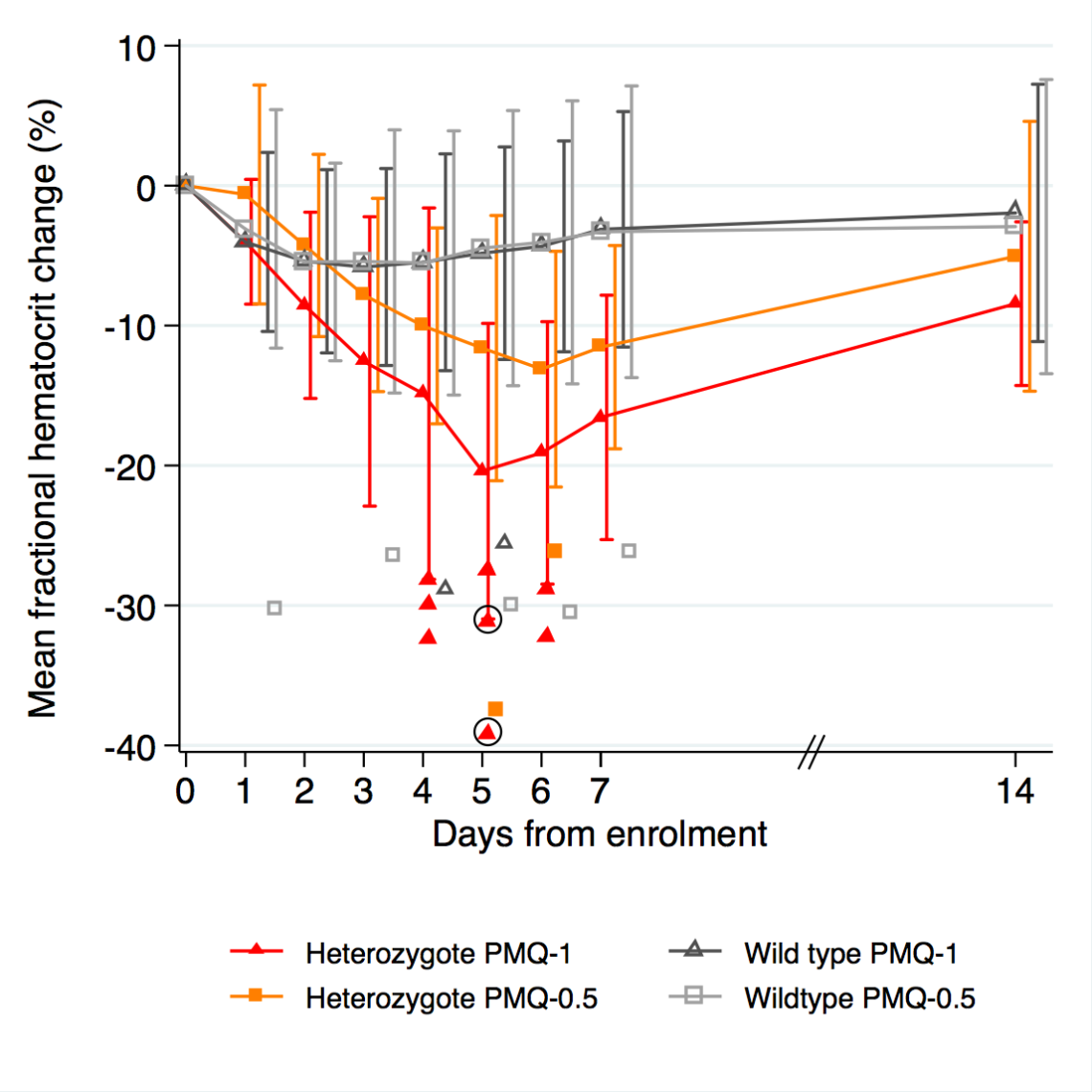
Primaquine in G6PD heterozygotes
Key publications
-
Haemolysis in G6PD Heterozygous Females Treated with Primaquine for Plasmodium vivax Malaria: A Nested Cohort in a Trial of Radical Curative Regimens
Journal article
Chu CS. et al, (2017), PLOS Medicine, 14, e1002224 - e1002224
-
Tafenoquine versus Primaquine to Prevent Relapse of Plasmodium vivax Malaria.
Journal article
Llanos-Cuentas A. et al, (2019), The New England journal of medicine, 380, 229 - 241
-
Malaria in children
Journal article
Crawley J. et al, (2010), The Lancet, 375, 1468 - 1481
-
Resolving the cause of recurrent Plasmodium vivax malaria probabilistically
Journal article
Taylor AR. et al, (2019), Nature Communications, 10
-
Chloroquine Versus Dihydroartemisinin-Piperaquine With Standard High-dose Primaquine Given Either for 7 Days or 14 Days in Plasmodium vivax Malaria
Journal article
Chu CS. et al, (2019), Clinical Infectious Diseases, 68, 1311 - 1319
-
Comparison of the Cumulative Efficacy and Safety of Chloroquine, Artesunate, and Chloroquine-Primaquine in Plasmodium vivax Malaria
Journal article
Chu CS. et al, (2018), Clinical Infectious Diseases, 67, 1543 - 1549
-
Management of relapsingPlasmodium vivaxmalaria
Journal article
Chu CS. and White NJ., (2016), Expert Review of Anti-infective Therapy, 14, 885 - 900
Recent publications
-
Evaluation of the Wondfo G6PD/Hb Test for glucose-6-phosphate dehydrogenase deficiency: preliminary performance, matrix equivalence, and usability.
Journal article
Green RK. et al, (2025), Malaria journal, 24
-
Submicroscopic malaria in pregnancy and associated adverse pregnancy events: A case-cohort study of 4,352 women on the Thailand–Myanmar border
Journal article
Gilder ME. et al, (2025), PLOS Medicine, 22, e1004529 - e1004529
-
High-dose primaquine reduces vivax relapses: time for change.
Journal article
Commons RJ. and Chu CS., (2025), The Lancet. Infectious diseases
-
A mixed methods study investigating factors affecting adherence to Plasmodium vivax malaria primaquine radical cure regimens among migrants along the Myanmar-Thailand border
Journal article
Ansari AT. et al, (2025), PLOS Global Public Health, 5, e0003615 - e0003615
-
Primaquine for uncomplicated Plasmodium vivax malaria in children younger than 15 years: a systematic review and individual patient data meta-analysis
Journal article
Commons RJ. et al, (2024), The Lancet Child & Adolescent Health, 8, 798 - 808